A certificate of analysis is issued by a Quality Assurance (QA) Department to a manufacturer or a seller of products. The QA department conducts various tests in the laboratories on the products, as required, to investigate different product-related features and characteristics to check if the quality standards have been met. These features and their standards represent the overall quality of the product.
This document certifies that the product quality and its specifications match with the required standards and hence, make it an acceptable product.
This certificate holds importance because:
- This certificate is like evidence of the quality that can be used by the companies to portray and market their image and products.
- Sometimes, government regulations enforce the ownership of such certificates. Otherwise, a business is not allowed to operate until it gets quality assurance from an authorized Quality Assurance department.
- It smoothens many governmental procedures, aids in product registration, and helps in gaining manufacturing patent rights.
- If an international transaction of import/export is involved in the manufacturing or selling of a product, this certificate of analysis may help ease and speed up the customs clearance of raw materials or the final product.
- This certificate may help in getting customers and increasing market share.
- It can help during lawsuits or cases filed by unsatisfactory clients.
The Quality Assurance Departments either design templates for various scenarios and product types or use the available templates from online sources or programs, such as Microsoft Word. In the latter option, the templates used have been professionally designed by the experts and the chances of mistakes are minimal. In addition, the costs, money, and efforts get saved, if the QA Department utilizes these readymade templates, as well as those, that can easily be customized as per the required details which further limits the hassle.
As there are various product types present and quality assurance can be sought by a manufacturer or a seller for different product specifications and features, the details included in the certificate of analysis vary for different countries, companies, and products. At the same time, generally, the following details (more or less similar) are included in almost all types of the certificates of Analysis:
- Date of issuance.
- Date of release.
- The validity of the certificate.
- Details of the Laboratory/company.
- Details of the authorized person with signature.
- Details of the manufacturer.
- Details of the supplier, if different from the manufacturer’s information.
- Details of the product, including name, code or ID, manufacturing date, batch number, expiry date, quantity produced, etc.
- Product receiving date, lab batch number, and quantity received.
- Data of the findings and comparison with the set and acceptable standards of quality.
- Certification of analysis and findings.
- Approval and acceptance of the product.
The QA department needs to have sound technology and laboratories for running the tests of quality checks.
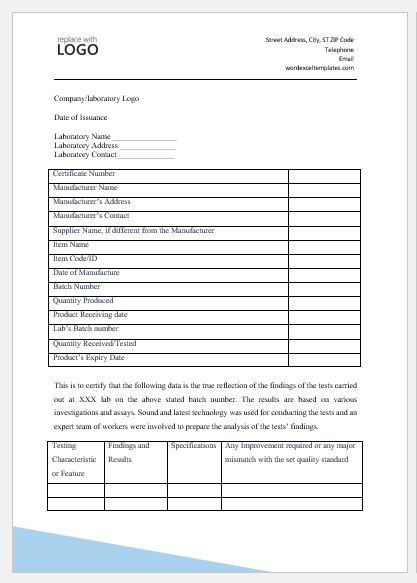
Sample Certificate of Analysis
Company/laboratory Logo
Date of Issuance
Laboratory Name ________________
Laboratory Address ______________
Laboratory Contact ______________
| Certificate Number | |
| Manufacturer Name | |
| Manufacturer’s Address | |
| Manufacturer’s Contact | |
| Supplier Name, if different from the Manufacturer | |
| Item Name | |
| Item Code/ID | |
| Date of Manufacture | |
| Batch Number | |
| Quantity Produced | |
| Product Receiving date | |
| Lab’s Batch number | |
| Quantity Received/Tested | |
| Product’s Expiry Date |
This is to certify that the following data is the true reflection of the findings of the tests carried out at ABC lab on the above-stated batch number. The results are based on various investigations and assays. Sound and the latest technology were used for conducting the tests and an expert team of workers was involved to prepare the analysis of the tests’ findings.
| Testing Characteristic or Feature | Findings and Results | Specifications | Any Improvement required or any major mismatch with the set quality standard |
The tests were initiated on the same day of receiving the shipment and the duration of testing procedures was two days. This certificate ascertains that the products had the required specifications and met the set standards of quality. No major improvements are needed. We approve this batch of the products and certify that our investigation before the shipment revealed satisfactory results.
All the above information is based on the supplier’s provided details, for which, if found incorrect, we hold no responsibility. We have used our best knowledge in conducting the tests and stating the above findings and we have attempted to avoid any incorrect or misleading information.
Date of Release_______________
Validity of certificate ___________
Lab Manager Name____________
Signature____________________
File: Word (.docx) 2007/+ and iPad Size 66 Kb | Download
License: ENERGY (Personal use only)
(Distribution) by Kate Elizabeth(CEO)
